|
|
The Single Helix
Chemical Element System data
for Periodic Table Applications
 The natural atoms can only be arranged
The natural atoms can only be arranged
with all element relationships perfectly
correct by spiralling every element
down a single helix on a 3D model,
groups aligned and elements contiguous.
 Flat periodic tables currently employ this
Flat periodic tables currently employ this
systemized data in the more convenient
2D tabular format necessary for easier
printing, study and work of Chemistry.
 The differences between flat periodic tables
The differences between flat periodic tables
and the three-dimensional system model
photographed to illustrate this principle in
the views at the right have been annotated
to the right of the photos.
 Swipe right to see more, and click on the
Swipe right to see more, and click on the
photo to enlarge, and again to return.

BACK
|
|
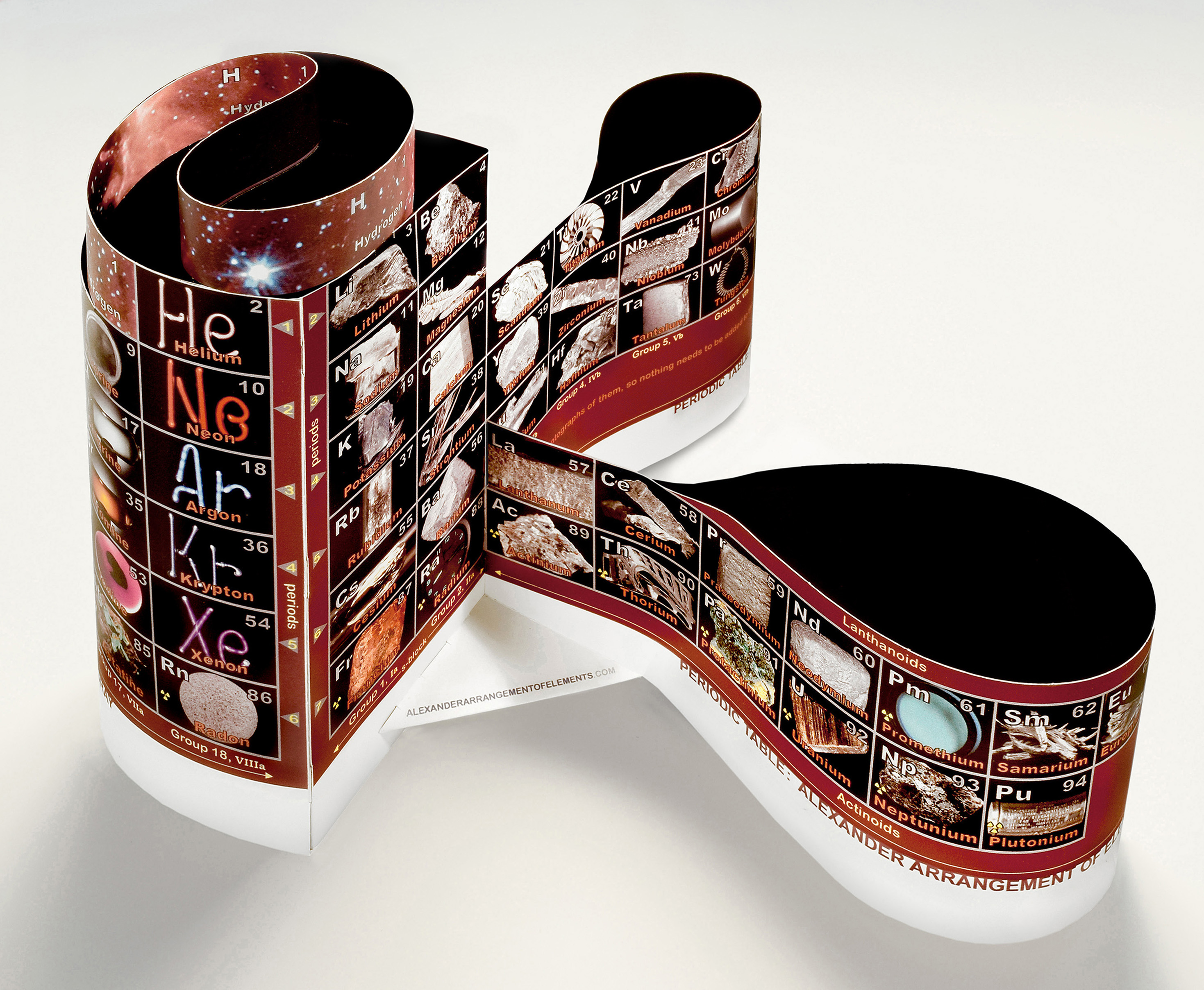
 The stretched and wrapped
Hydrogen box (originating from
nothing at the Big Bang) unites
with Helium, completing the first period,
atop the 6 period Main Group
element tower at the left.
The stretched and wrapped
Hydrogen box (originating from
nothing at the Big Bang) unites
with Helium, completing the first period,
atop the 6 period Main Group
element tower at the left.
 While the F- & D-blocks (right)
are each independent, connecting
with each other plus to both S-block's
Group IIa bordering on the single vertical
location I call the neXus, results in the
Atomic Numbers of the Element Line
being continuous from start to finish,
while keeping all columns intact as well.
While the F- & D-blocks (right)
are each independent, connecting
with each other plus to both S-block's
Group IIa bordering on the single vertical
location I call the neXus, results in the
Atomic Numbers of the Element Line
being continuous from start to finish,
while keeping all columns intact as well.
 Instead of being at the edges of
the periodic table, both the ends
and beginnings of the periods are
together - greatly improving
trends teaching - and that junction
is clearly defined by the period
arrows pointing to the last and
next periods on the corner
bars between Groups 18 & 1.
Instead of being at the edges of
the periodic table, both the ends
and beginnings of the periods are
together - greatly improving
trends teaching - and that junction
is clearly defined by the period
arrows pointing to the last and
next periods on the corner
bars between Groups 18 & 1.
|

|
 Hydrogen's unique extended databox
makes explicit the concept that
Period 1 is quite different from those
following, and by circling over
the Main Group, ready compounding
with those elements is implied.
Hydrogen's unique extended databox
makes explicit the concept that
Period 1 is quite different from those
following, and by circling over
the Main Group, ready compounding
with those elements is implied.
 In the Big Bang, Hydrogen, first,
then Helium, then Lithium are
thought to have been realized
first, the same order illustrated
from top down in this model,
rather than being separated on
a common periodic table.
In the Big Bang, Hydrogen, first,
then Helium, then Lithium are
thought to have been realized
first, the same order illustrated
from top down in this model,
rather than being separated on
a common periodic table.
 Explicit in the Periodic Law,
numerically contiguous elements
are only available in 3D models like the
Alexander Arrangement, shown at
left by the slant of P-block databoxes.
This patented feature is what allows
period ends to physically connect
with the next period's first element
Explicit in the Periodic Law,
numerically contiguous elements
are only available in 3D models like the
Alexander Arrangement, shown at
left by the slant of P-block databoxes.
This patented feature is what allows
period ends to physically connect
with the next period's first element
- a key trends factor that flat periodic
tables sacrifice in exchange for all
elements to be visible at one time.
|

|
 Seeing all element information at once
on a convenient surface is vital to the
doing of chemistry, but for the initial
introduction and understanding of the
correct relationships among both
elements and blocks, models of the
Alexander Arrangement of Elements
are required to show Mendeleyev's
Periodic Law as well as the top down S P D F
subshell order.
Seeing all element information at once
on a convenient surface is vital to the
doing of chemistry, but for the initial
introduction and understanding of the
correct relationships among both
elements and blocks, models of the
Alexander Arrangement of Elements
are required to show Mendeleyev's
Periodic Law as well as the top down S P D F
subshell order.
 Large databoxes names, symbols,
and numbers help Howard Gardner's
verbal-linguistic students, while Theodore Gray's
'prior knowledge' photography appeals
to those favoring naturalistic competencies.
Large databoxes names, symbols,
and numbers help Howard Gardner's
verbal-linguistic students, while Theodore Gray's
'prior knowledge' photography appeals
to those favoring naturalistic competencies.
 At the same time, the physicality
of this model requires manipulation -
attractive to the bodily-kinesthetic as
well as visual-spatial intelligences -
adding these students to others for
whom the common flat table is a
disappointment or deterrent.
At the same time, the physicality
of this model requires manipulation -
attractive to the bodily-kinesthetic as
well as visual-spatial intelligences -
adding these students to others for
whom the common flat table is a
disappointment or deterrent.
|

|

 Instead of Main Group blocks
being pushed apart by the
transition metals, common in
periodic tables, the other block
loops being pinched where they
join between Groups 2 & 3
avoid that distortion: seen here in the center, where all element numbers are sequential - the neXus.
Instead of Main Group blocks
being pushed apart by the
transition metals, common in
periodic tables, the other block
loops being pinched where they
join between Groups 2 & 3
avoid that distortion: seen here in the center, where all element numbers are sequential - the neXus.
 Feeling and seeing the natural
beauty of science, which are
embodied in this model -
either as a great periodic
table or a model of the
reality of the atoms/elements
systematized according to
the Periodic Law - provides
greater understanding
and appreciation of Science, so important for learning!
Feeling and seeing the natural
beauty of science, which are
embodied in this model -
either as a great periodic
table or a model of the
reality of the atoms/elements
systematized according to
the Periodic Law - provides
greater understanding
and appreciation of Science, so important for learning!
 This Alexander Arrangement of
Elements model requires
hands-on to see all elements -
adding respect for the
Periodic Law and a glimpse
of reality.
This Alexander Arrangement of
Elements model requires
hands-on to see all elements -
adding respect for the
Periodic Law and a glimpse
of reality.
|
|

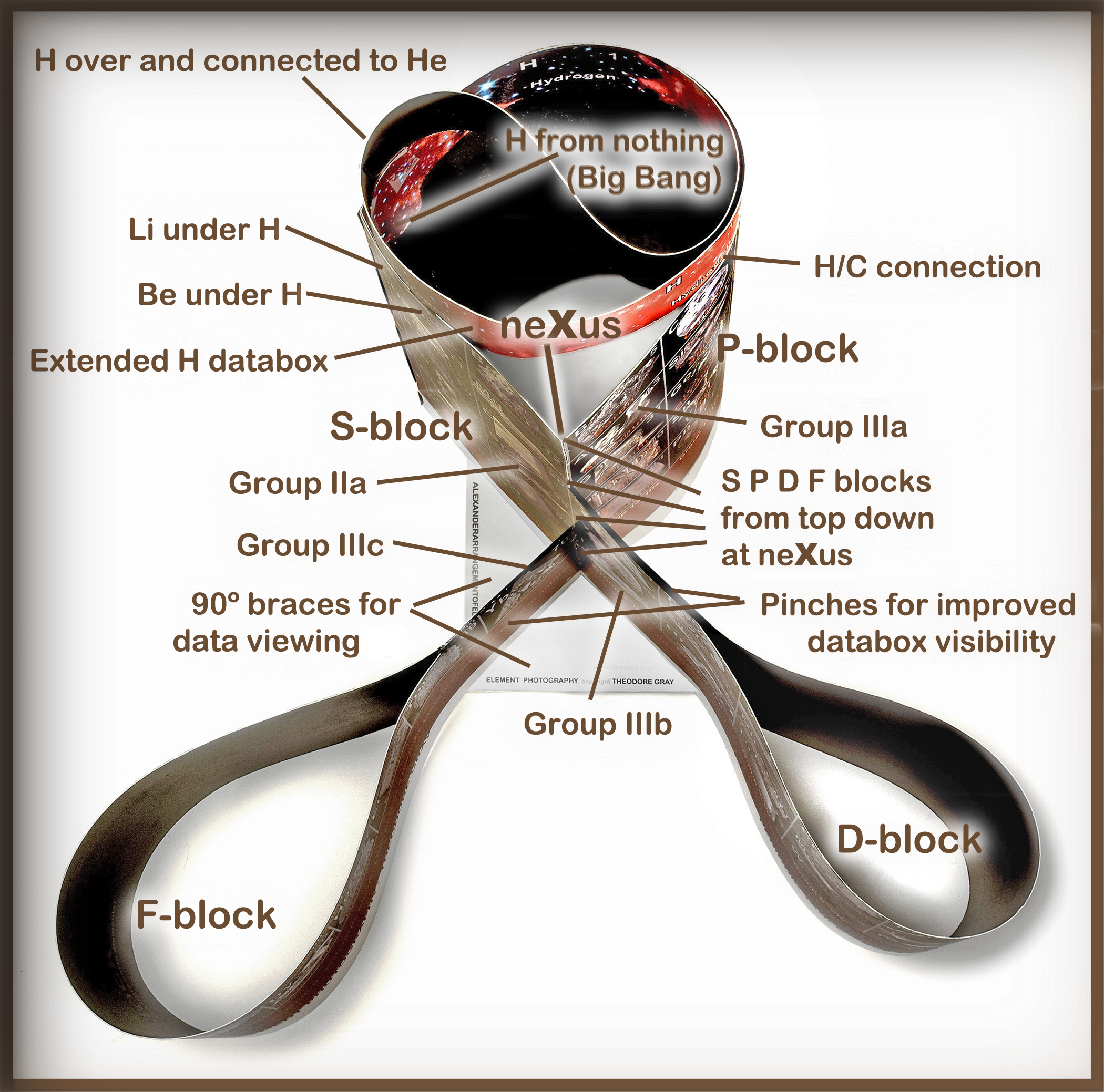
 The unique form and function of the
Alexander Arrangement of Elements
shows best from the top.
The unique form and function of the
Alexander Arrangement of Elements
shows best from the top.
 The extended H databox originates
from nothing (like the Big Bang)
inside the Main Group tower, rising
clockwise to touch on He, Li, and Be,
and descends in the P-block (where
the Alexander downslant takes place
in all periods) to rest against He.
The extended H databox originates
from nothing (like the Big Bang)
inside the Main Group tower, rising
clockwise to touch on He, Li, and Be,
and descends in the P-block (where
the Alexander downslant takes place
in all periods) to rest against He.
 The crossover of the blocks at the
neXus shows the attachment of S-
block's Group IIa to the first elements
(Groups IIIa,b,c) of all other blocks.
The crossover of the blocks at the
neXus shows the attachment of S-
block's Group IIa to the first elements
(Groups IIIa,b,c) of all other blocks.
 This Alexander Arrangement of Elements
model of the Chemical Element System
Involves a student more thoroughly -
emotionally, mentally, and physically - than any 2D chart,
for a great advance in understanding.
This Alexander Arrangement of Elements
model of the Chemical Element System
Involves a student more thoroughly -
emotionally, mentally, and physically - than any 2D chart,
for a great advance in understanding.
|

|
 More about the
More about the
features and educational
capabilities of this kit
for an illustrated model
of the Periodic Law
may be found at
3D Periodic Table.
|

|





